

Mesenchymal Stem Cell Therapy/Bone Marrow Aspirate/Progenitor CellsĪetna considers the use of mesenchymal stem cell therapy (e.g., AlloStem, Osteocel, Osteocel Plus, Ovation, Regenexx, and Trinity Evolution) and/or the use of progenitor cells experimental and investigational for all orthopedic applications including repair or regeneration of musculoskeletal tissue, osteochondritis dissecans, spinal fusion, and bone nonunions because there is insufficient evidence to support its use for these indications, especially its safety and long-term outcomes.Īetna considers bone marrow injections medically necessary in the treatment of bone cysts (unicameral/simple).Polymethylmethacrylate (PMMA) or Calcium Sulfate (e.g., Osteoset Resorbable Mini-Bead) Antibiotic BeadsĪetna considers PMMA antibiotic beads or calcium sulfate antibiotic beads (e.g., Osteoset Resorbable Mini-Bead) medically necessary for use in conjunction with intravenous antibiotics in the treatment of chronic osteomyelitis.Allograft materials that are 100% bone are considered medically necessary for these indications regardless of the shape of the implant. Allograft for Spinal Fusion and Osteochondral DefectsĪetna considers cadaveric allograft and demineralized bone matrix medically necessary for spinal fusions and for filling osteochondral defects (bone void fillers).Porcine Intestinal Submucosa Surgical MeshĪetna considers a surgical mesh composed of porcine intestinal submucosa experimental and investigational because its clinical value in rotator cuff repair surgery, repair of anorectal fistula, and for other indications has not been established.
#INFUSE BONE SKIN#
See also CPB 0244 - Skin and Soft Tissue Substitutes (stating that autologous platelet-rich plasma, autologous platelet gel, and autologous platelet-derived growth factors (e.g., Procuren) are considered experimental and investigational for chronic wound healing).
#INFUSE BONE PRO#
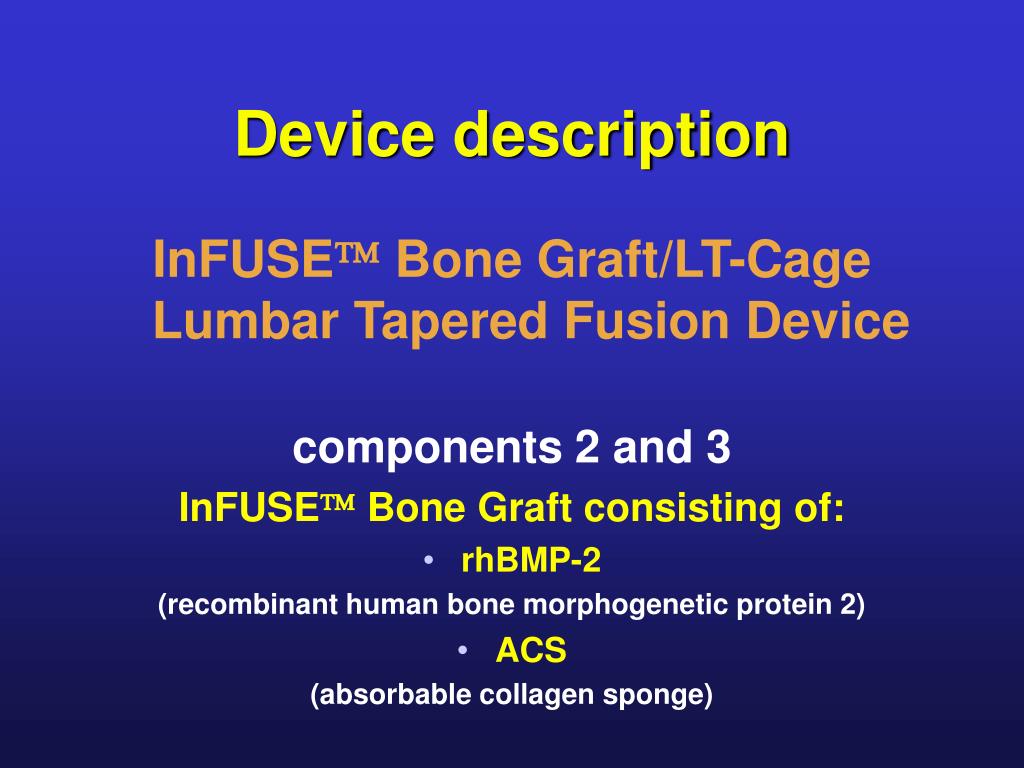
Pro Osteon Porous Hydroxyapatite Bone Graft SubstituteĪetna considers the Pro Osteon Porous Hydroxyapatite Bone Graft Substitute experimental and investigational for repair of metaphyseal fracture defects or repair of long bone cyst and tumor defects, because it has not been shown to be more effective than autograft or cadaveric allograft for these indications.Īetna considers the Pro Osteon Bone Graft Substitute experimental and investigational for use in spinal fusion, epiphyseal fractures or other indications because its effectiveness for these indications has not been established.Īetna considers the use of platelet-rich plasma, alone or in conjunction with bone grafting materials, experimental and investigational for augmentation procedures (e.g., for dental implants and for the floor of the maxillary sinus) or indications (e.g., soft tissue injuries) other than thrombocytopenia because its effectiveness has not been established.Note: The INFUSE Bone Graft is also known as bone morphogenic, or morphogenetic protein-2, BMP-2. The INFUSE Bone Graft is considered medically necessary for treating skeletally mature persons with acute, open tibial shaft fractures that have been stabilized with intramedullary nail fixation after appropriate wound management, when INFUSE Bone Graft is applied within 14 days after the initial fracture.Īetna considers the INFUSE Bone Graft experimental and investigational for all other indications, including its use in ankle fusions and cervical fusions, because its effectiveness for indications other than the ones listed above has not been established. INFUSE Bone Graft and device is to be implanted via an anterior (ALIF) or lateral (OLIF, DLIF, XLIF or LLIF) approach.INFUSE Bone Graft is to be used with a cage (for example, the MedtronicTitanium Threaded Interbody Fusion Device, the LT-CAGE Lumbar Tapered Fusion Device, or the INTER FIX or INTER FIX RP Threaded Fusion Device) and.The member meets medical necessity criteria for lumbar spinal fusion in CPB 0743 - Spinal Surgery: Laminectomy and Fusion and.INFUSE Bone Graft (Bone Morphogenic Protein-2)Īetna considers the INFUSE Bone Graft medically necessary for lumbar spinal fusion procedures in skeletally mature persons who meet the following criteria:.

Number: 0411 Table Of Contents Policy Applicable CPT / HCPCS / ICD-10 Codes Background References


 0 kommentar(er)
0 kommentar(er)
